X-Ray Photoelectron Spectroscopy (XPS, ESCA)
The surface analysis technique called X-Ray Photoelectron Spectroscopy (XPS) is also known as ESCA, electron spectroscopy for chemical analysis. XPS is chosen when the samples are insulating or when chemical state information is desired. XPS analysis is usually conducted in two parts. During the first portion, a complete surface elemental analysis is obtained. XPS detects atomic number range 3-92, within 50Å of the surface. There is good light element sensitivity, with a detectability limit of approximately 0.1 atomic percent. The second portion of a typical XPS experiment is used to determine the chemical binding energy of the surface elements. This portion is the true strength of XPS in that the binding energy furnishes some information about compounds present on the surface. Argon profiling is also available to determine concentration as a function of depth.
XPS Features Include
- Elemental surface composition from atomic number 3-92
- 10-50Å analysis depth
- 10µm spatial resolution
- Conductors and insulators can be analyzed
- Mapping
- Depth profiling with C-60 beam for organic materials
- Depth profiling with argon ion beam for inorganic materials
- Large sample size
Material issues analyzed with XPS include surface segregation, corrosion, semiconductor & electrical contact contamination, surface cleanliness, paint & coating delamination, and intergranular fracture.
Coating Failure
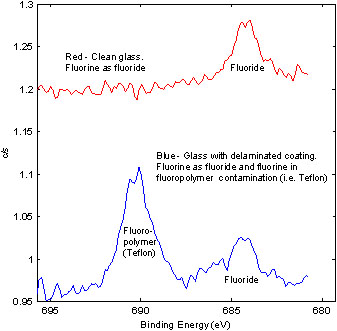
XPS is well suited to the study of coating failures. Analysis of the exposed material interface delineates the true location of the separation. Comparison of these surfaces to the bulk materials often shows the presence of contaminants.
In this example, XPS determined that a fluoride-containing glass surface was contaminated with a high level of Teflon®, preventing ultimate coating adhesion. The base glass contained fluoride compounds. Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer (most well known by the DuPont brand name Teflon®) with many applications. XPS can easily differentiate between the fluorine states of simple fluoride and fluoropolymer.
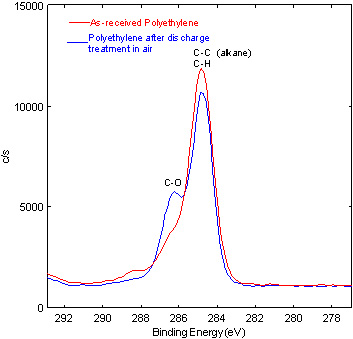
carbon oxidation state of untreated and treated polyethylene after discharge treatment in air. Shifting in the carbon XPS peaks indicate different binding states are present. The as-received polyethylene is predominantly alkane C-C carbon. Carbon-oxygen bonding is clearly present after the discharge process.
Polymer Technology
XPS has many applications in polymer technology. One application is the study of polymer film before and after surface treatment processes, including plasma treatment and corona discharge treatment. The data compare the carbon oxidation state of untreated and treated polyethylene after discharge treatment in air. Shifting in the carbon XPS peaks indicate different binding states are present. The as-received polyethylene is predominantly alkane C-C carbon. Carbon-oxygen bonding is clearly present after the discharge process.