Stainless steel is defined as a steel alloy with a minimum of 10.5 weight percent chromium. Although stainless steel does not generally corrode as easily as ordinary steel, different grades are necessary for different environments. For example, several manufacturing groups (including semiconductor and pharmaceutical) have stringent requirements for stainless steel surfaces. The corrosion-resistant properties of stainless steel are due to the formation of a passive chromium oxide layer. Although this layer forms naturally when the material is exposed to the atmosphere, additional chemical treatments (passivation) can improve the relative amount of chromium at the surface. The surface chemistry changes due to passivation occur within the outer ~50Å of the surface. In order to measure these changes, surface analytical tools such as x-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES) are necessary. One of the stainless steel measurements that is made using Auger and XPS is the near-surface chromium/iron (Cr/Fe) ratio.

Effect Of Chromium/Iron Ratio
A sample of corrugated 304L panel was exposed to a chloride-containing environment. The chromium/iron ratio and the sample appearance are shown. A lower Cr/Fe ratio results in more corrosion.

Good appearance
Cr/Fe = 2.1
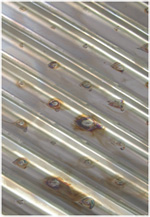
Sporadic corrosion
Cr/Fe = 0.6
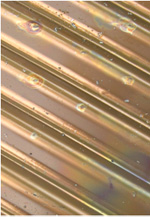
Substantial corrosion
Cr/Fe = 0.3
Specific procedures have been developed by Material Interface to record the surface chemistry of these samples. The analysis can also be conducted according to specifications in standards published by Sematech and SEMI*.
Analytical Steps
When stainless steel is analyzed with XPS or Auger, four steps are included:
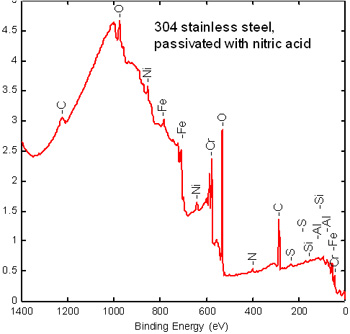
Step 1. Stainless steel XPS survey spectrum
A survey scan determines the overall elemental composition of the surface from atomic numbers 3-92. This determines the composition of the passive layer elemental composition and surface Cr/Fe ratio. High C and O are typical on metals exposed to the air. Other elements are present due to processing and to handling.
Step 2. Chemical State Information
If the analysis is conducted with XPS, the major elements detected (usually carbon, oxygen, silicon, chromium, iron, nickel, and molybdenum) are analyzed in high energy resolution mode to determine their binding energies and then to make some inferences about the compounds present on the surface. This measurement determines the relative amounts of metals versus oxides for chromium, iron, and nickel and the distribution of organic carbon states.
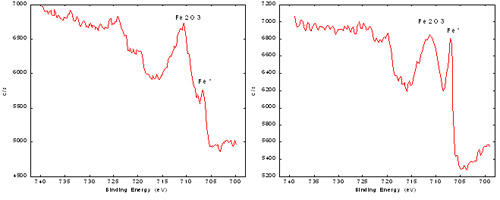
The right iron spectrum was obtained from a sample that had undergone experimental passivation, while the left iron spectrum was from a standard passivation treatment. XPS demonstrated there is more metallic iron present on the surface of the experimentally passivated material, resulting in subsequent rust formation.
Step 3. Depth Profile
The third step in the analysis includes a depth profile to determine the concentration of the primary elements (C, O, Cr, Fe, and Ni) as a function of depth. This also yields the Cr/Fe ratio as a function of depth and the oxide thickness. As an example, for the polished sample (below, left graph), the iron concentration is always higher than the chromium concentration. For the passivated sample (below, right graph), the concentration of chromium is greater than the concentration of iron in the near-surface region.
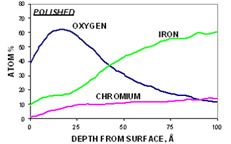
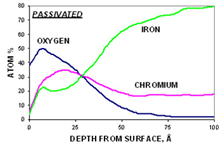
Step 4. After-profiling composition of all elements (for quantitative comparison)
Susan Kerber of Material Interface has been actively involved with ASTM International on the development of a new stainless steel passivation standard. The new standard is currently undergoing round-robin testing. Please contact our office for additional information or for a quote.
What is Rouge and how is it removed?
Rouge is a surface deposit and discoloration on stainless steel surfaces, with various colors and textures. It generally appears after exposure of stainless to high-purity water and steam at elevated temperatures and often reappears after removal. Rouge is composed of various chromium and iron oxides. These forms are designated as Class I, Class II, and Class III Rouge. Class I Rouge is orange/reddish-brown and is primarily iron oxides and hydroxide. It is only loosely adhered to the base metal and is therefore migratory. The base alloy underneath the rouge is not affected by this deposit. It can often be removed by passivating processes. Class II Rouge is typical of rust formation, often from the presence of halides like chloride. It is active surface corrosion with a low chromium/iron ratio. Removal often requires abrasion and re-polishing. Class III Rouge forms in high temperature situations, including clean steam. It is dark blue to black in color and is chemically very stable. It contains iron oxide in magnetite form and is tightly adhered to the base alloy. In some cases, strong acids can remove this layer. In other cases, the surfaces much be mechanically repolished.
* Sematech, 2706 Montopolis Drive, Austin, TX 78741, Specifications 90120403B-STD and 91060573B-STD SEMI Global Headquarters, 3081 Zanker Road, San Jose, CA 95134, Specifications F30-0306 (XPS), F72-1102 (Auger), F73-1102 (SEM).
