 Oxidation of stainless steel, commonly called rouging in the pharmaceutical industry, can be studied with Auger electron spectroscopy and X-ray diffraction.
Oxidation of stainless steel, commonly called rouging in the pharmaceutical industry, can be studied with Auger electron spectroscopy and X-ray diffraction.
- EDS indicated iron oxide formation
- What is Cr/Fe ratio between crystals? –Auger analyzed and determined Cr/Fe of substrate = 3.1 (not likely an original passivation problem)
- What iron oxide form is present? –XRD documented Fe2O3

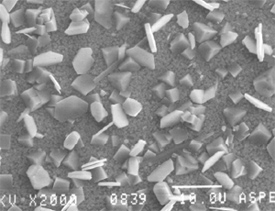
SEM image
|

O
|

Fe
|

Cr
|
Auger Images and Maps
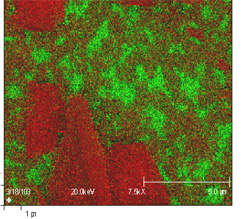
Composite Auger map
Red = Fe; Green = Cr
Red = Fe; Green = Cr
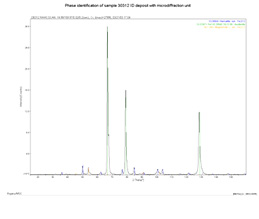
X-ray diffraction for Fe2O3 confirmation
(Auger: Fe2O2.96)
(Auger: Fe2O2.96)
Questions to Answer
- Do the iron oxide particles originate from a foreign source? Are deposits easily cleaned? If so, the passive layer of the stainless steel is likely uncompromised.
- If the iron oxides are formed in the location where they are found, there are typically lapses of the passive layer. These deposits can be removed by mechanical means or by light acid etching.
- If the rouge turns gray or black with exposure to elevated temperature, there is a chemical reaction occurring. These oxides are relatively stable and can be removed chiefly by mechanical means or a strong acid.
